FULL APPROVAL of Covid Vaccine Coming As Soon as Monday According to Multiple Reports
Mario Tama/Getty Images
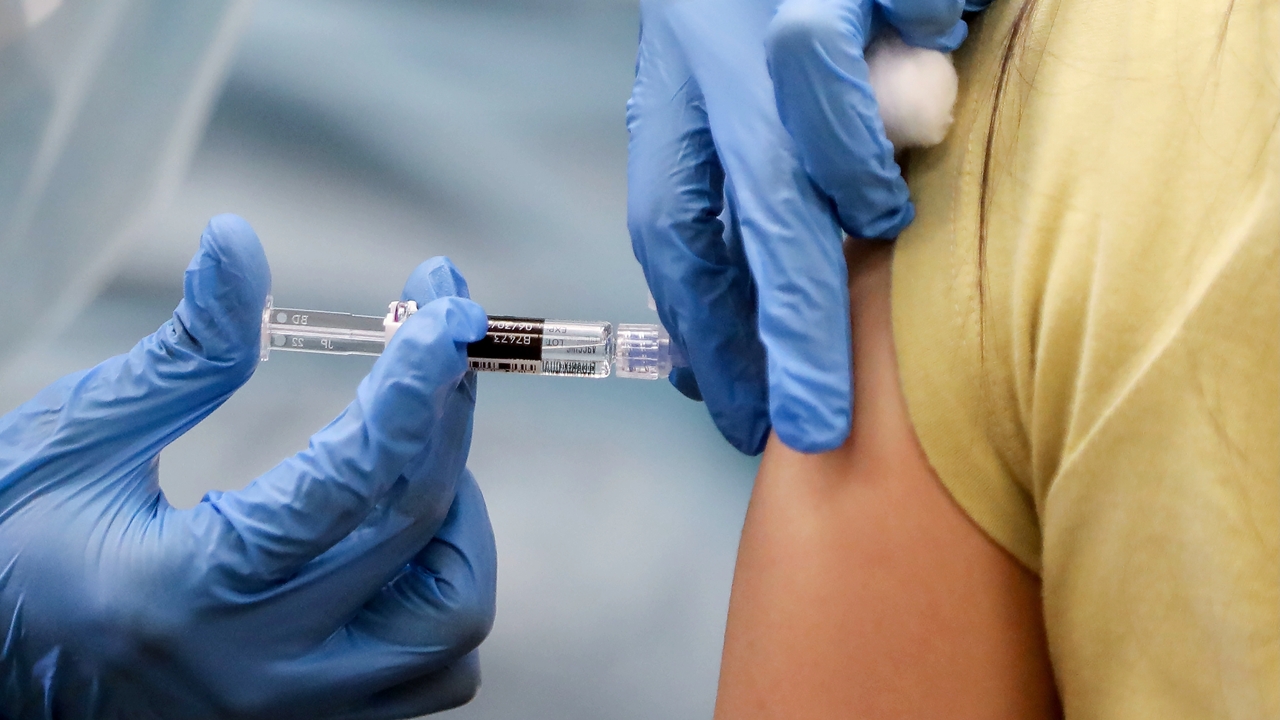
The Food and Drug Administration (FDA) is set to grant full approval to Pfizer’s coronavirus vaccine as soon as Monday, according to reports from multiple outlets.
The nationwide vaccination effort has been proceeding under Emergency Use Authorizations, but the FDA is on the precipice of granting full approval. According to The New York Times‘ Noah Weiland and Sharon LaFraniere, Monday is the target date:
The Food and Drug Administration is pushing to approve Pfizer-BioNTech’s two-dose Covid-19 vaccine on Monday, further expediting an earlier timeline for licensing the shot, according to people familiar with the agency’s planning.
Regulators were working to finish the process by Friday but were still working through a substantial amount of paperwork and negotiation with the company. The people familiar with the planning, who were not authorized to speak publicly about it, cautioned that the approval might slide beyond Monday if some components of the review need more time.
And The Washington Post has similar reporting from an equal number of sources:
The Food and Drug Administration is expected to grant full approval to Pfizer-BioNTech’s coronavirus vaccine in the coming days, according to four people with knowledge of the plans.
If approved, the vaccine would be the first in the United States to receive full licensure, and it could result in private businesses issuing a new wave of vaccine mandates.
Vaccine hesitancy has been a consistent obstacle to achieving herd immunity, and has fueled the Delta surge, but full FDA approval has been shown to be an enticement for those who have been on the fence about getting vaccinated.
New: The Mediaite One-Sheet "Newsletter of Newsletters"
Your daily summary and analysis of what the many, many media newsletters are saying and reporting. Subscribe now!





Comments
↓ Scroll down for comments ↓